Titanium, the 7th most abundant metal in the earth’s crust, has highly attractive physico-chemical and mechanical characteristics. This transition metal is light, corrosion-resistant and offers good mechanical properties.
Here are the principal arguments which have made and still make titanium the material of choice in numerous industrial applications.
Excellent resistance to erosion and corrosion
Titanium is a transition metal which, in the presence of oxygen, spontaneously forms an insulating and protective layer of titanium oxide, TiO2. This ability for self-passivation classifies it in the family of ‘valve’ metals.
The layer of oxide formed on the surface of the titanium is insulating and constitutes a physical barrier against corrosive substances (oxygen or chlorine for example).
Good mechanical properties at up to 600°C and good adherence to coatings
Properties which make titanium a base material of choice for the manufacture of composite anodes.
High electrical and thermal conductivity
Characteristics which justify the use of titanium as a material for electrodes.
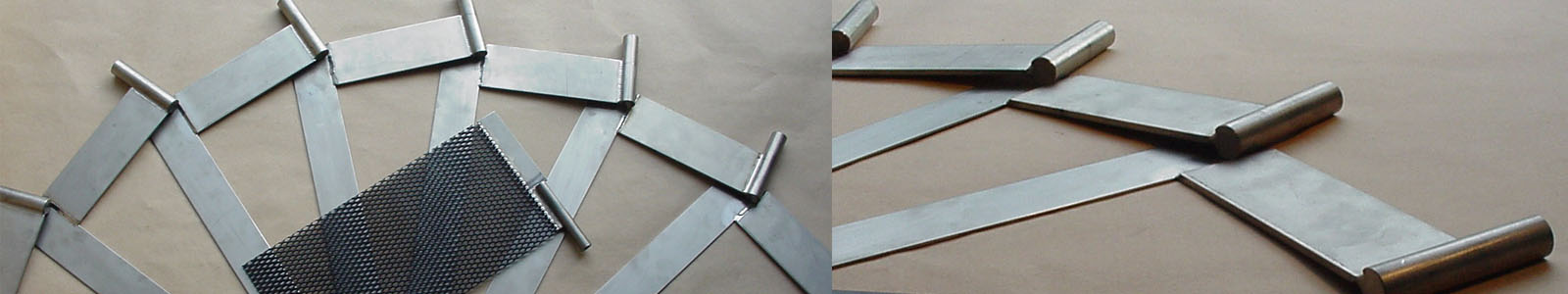
Diverse range of possible configurations
The malleability of titanium means easy machining so titanium can be found in the form of sheets, mesh, cord, tubes, wire, etc.
Lightweight material
The low density of titanium means that it is classified as a light metal. With a density of 4.51 titanium is about 40% lighter than steel, whose density is around 8–9 according to grade.